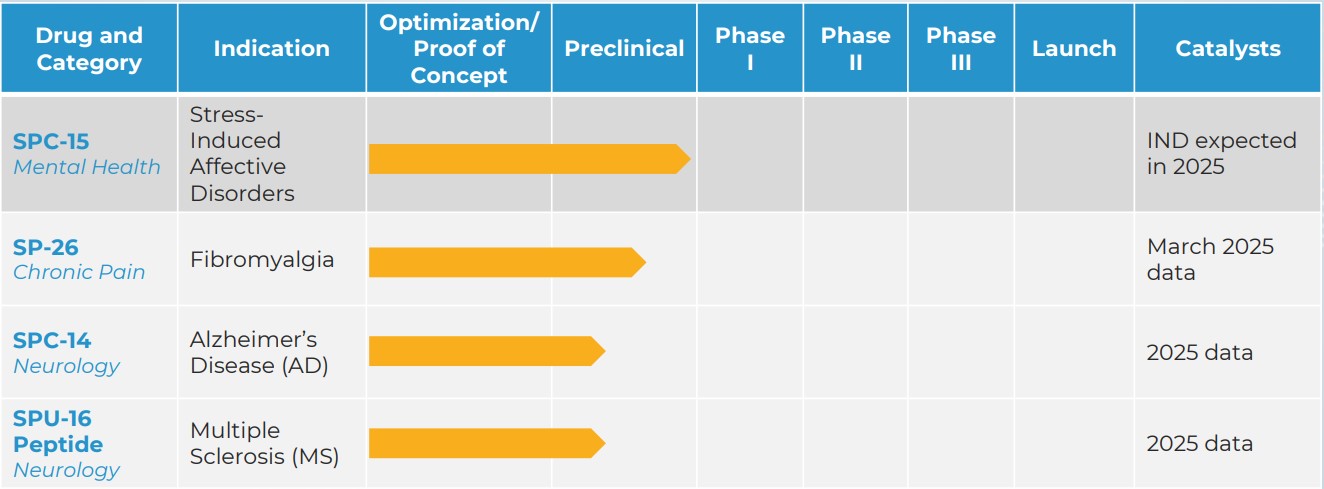
Pipeline
Showing promise in a range of illnesses and diseases.
Silo Pharma believes in following the science to identify the best application for a therapeutic rather by targeting indications with the biggest need and selecting the best route of delivery for patients. Our current assets have shown promise in a range of illnesses and diseases, including PTSD, Stress-Induced Psychiatric Disorders and Alzheimer’s Disease.